| 教育与研究经历: 2004年9月-2008年6月 四川大学 化学学院 理学 学士 2008年9月-2013年6月 四川大学 化学学院 理学 博士 2013年7月-至今 3499cc拉斯维加斯 化学化工学院 历任讲师、副教授、教授 主讲课程: 本科生:《有机化学》理论课/主干课 研究生:《有机波谱分析》必修课/专业主干课
研究领域与兴趣:
1、手性亲核催化剂的设计及应用
2、手性吡啶配体的设计及应用 3、手性核苷的不对称合成 主持的科研项目: 国家优秀青年科学基金、国家自然科学基金面上项目(已结项)、青年项目(已结项)等 获奖:
1、在中华全国总工会、教育部联合主办的第六届全国高校青年教师教学竞赛中荣获理科组二等奖(2023.04) 2、“催化不对称[8+2]环加成反应构建环庚三烯并吡咯烷-3,3'-吲哚酮,《化学学报》,2014年7月,谢明胜,武晓霞,王刚,林丽丽,冯小明*” 获得“第三届中国科协优秀科技论文”(2018年10月) 3、第二届“卢锦梭师德楷模奖教金” 4、河南省优秀教师(2023年8月) 5、河南省教育系统教学技能竞赛一等奖(高校理科组第二名)(2021年10月) 6、河南省教学标兵(2021年10月) 7、新乡市市长教育质量奖——名教师质量奖(2021年9月) 8、3499cc拉斯维加斯第六届青年教师课堂教学比赛一等奖(理科组第一名)(2021年7月) 9、3499cc拉斯维加斯第六届青年教师课堂教学十佳教师(2021年7月) 10、2018-2020学年度3499cc拉斯维加斯“优秀教师”荣誉称号
11、2022年度3499cc拉斯维加斯“五四青年奖章” 12、2016年度3499cc拉斯维加斯“新闻宣传先进个人”荣誉称号 代表性论文: [1] Tao Wei, Han-Le Wang, Yin Tian*, Ming-Sheng Xie*, Hai-Ming Guo*, Enantioselective construction of stereogenic-at-sulfur(IV) centers via catalytic acyl transfer sulfinylation, Nature Chemistry, 2024, 16, 1301-1311.

[2] Hai-Xia Wang, Chun Yang, Bai-Yu Xue, Ming-Sheng Xie*, Yin Tian*, Cheng Peng, and Hai-Ming Guo* Design of C1-symmetric tridentate ligands for enantioselective dearomative [3 + 2] annulation of indoles with aminocyclopropanes, Nat. Commun. 2023, 14, 2270. 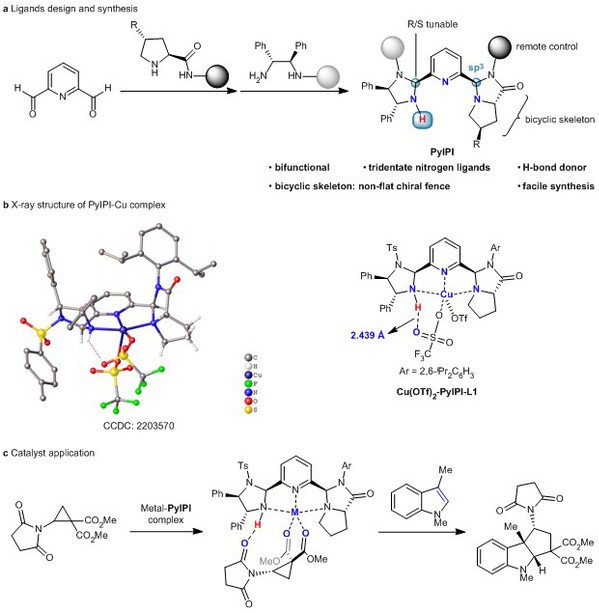
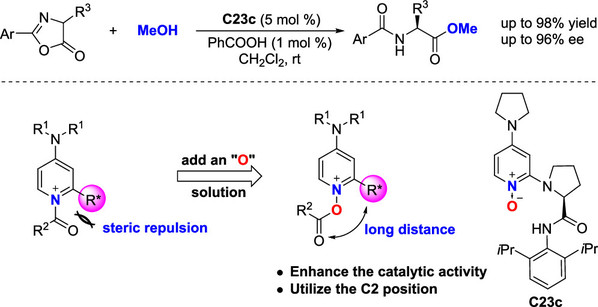
[3] Ming-Sheng Xie*, Bin Huang, Ning Li, Yin Tian*, Xiao-Xia Wu, Yun Deng, Gui-Rong Qu, and Hai-Ming Guo* Rational Design of 2-Substituted DMAP-N-oxides as Acyl Transfer Catalysts: Dynamic Kinetic Resolution of Azlactones.J. Am. Chem. Soc. 2020, 142(45), 19226-19238.(Highlight by Synfacts和X-MOL)
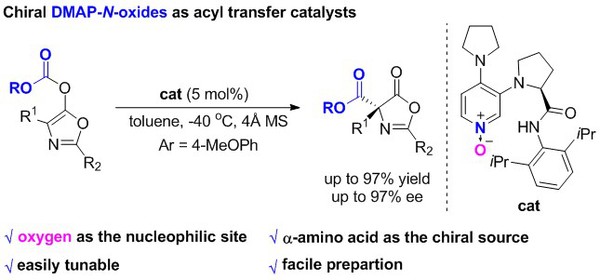
[4] Ming-Sheng Xie,* Ye-Fei Zhang, Meng Shan, Xiao-Xia Wu, Gui-Rong Qu, and Hai-Ming Guo*, Chiral DMAP-N-Oxides as Acyl Transfer Catalysts: Design, Synthesis, and Application in Asymmetric Steglich Rearrangement, Angew. Chem. Int. Ed. 2019, 58(9), 2839-2843 (Highlight by 《有机化学》和X-MOL).
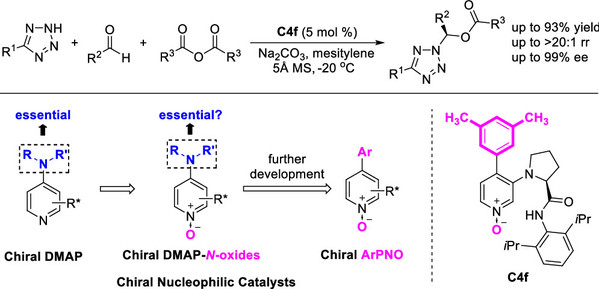
[5] Ming-Sheng Xie*, Meng Shan, Ning Li, Yang-Guang Chen, Xiao-Bing Wang, Xuan Cheng, Yin Tian*, Xiao-Xia Wu, Yun Deng, Gui-Rong Qu, and Hai-Ming Guo* Chiral 4-Aryl-Pyridine-N-oxide Nucleophilic Catalysts: Design, Synthesis, and Application in Acylative Dynamic Kinetic Resolution. ACS Catalysis, 2022, 12, 877-891.
[6] Ming-Sheng Xie*, Ning Li, Yin Tian*, Xiao-Xia Wu, Yun Deng, Gui-Rong Qu, and Hai-Ming Guo* Dynamic Kinetic Resolution of Carboxylic Esters Catalyzed by Chiral PPY-N-oxides: Synthesis of Nonsteroidal Anti-Inflammatory Drugs and Mechanistic Insights, ACS Catalysis, 2021, 11, 8183-8196.
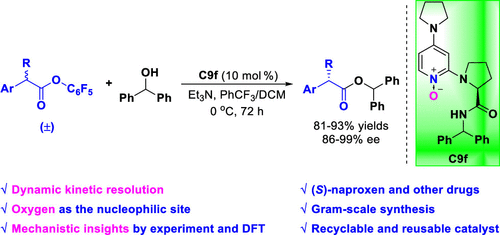
[7] Heng-Bin Yu, Yang-Guang Chen, Yin Tian*, Ming-Sheng Xie*, Hai-Ming Guo*, Nickel(II)-Catalyzed Asymmetric Inverse-Electron-Demand Diels–Alder Reaction of 2-Pyrones with Styrenes and Indenes, ACS Catalysis, 2024, 14, 11, 8930-8938.
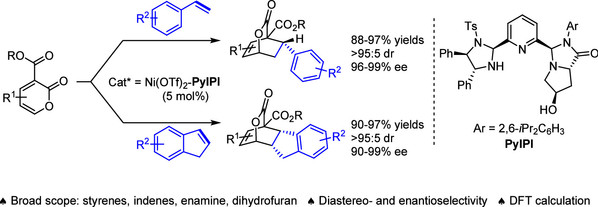
[8] Xing-Yu Wang, Xiao-Bing Wang, Yin Tian*, Cheng Peng, Ming-Sheng Xie*, Hai-Ming Guo*, Cobalt-Catalyzed Asymmetric Dearomative [3 + 2] Annulation of Quinolines, Isoquinolines, and Pyridines, ACS Catal. 2023, 13, 17, 11528-11540.
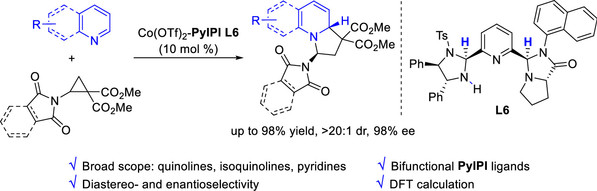
[9] Chen-Ying Hou, Chun Yang, Yin Tian*, Ming-Sheng Xie*, Hai-Ming Guo*, Achiral Counteranion-Induced Reversal of Enantioselectivity in Ni(II)-Catalyzed Friedel–Crafts Alkylation/Annulation of 2-Naphthols, Org. Lett., 2024, 26, 30, 6390-6395.
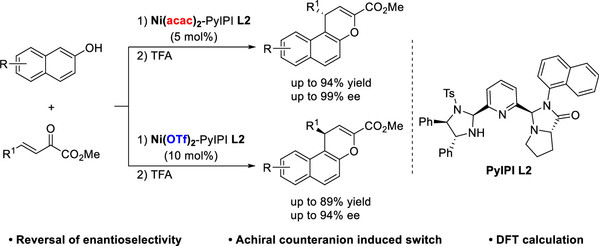
[10] Yu-Lin Gao, Yang Yang, Chen Wu, Ming-Sheng Xie*, Hai-Ming Guo*, Chemoselectivity Switch between Enantioselective [2,3]-Wittig Rearrangement and Conia-Ene-Type Reactions of Propargyloxyoxindoles, Chem. Eur. J. 2024, 30, 56, e202402556. 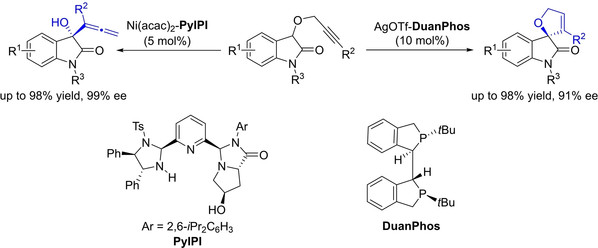
[11] Ru-Lin Luo, Xiao-Bing Wang, Ming-Sheng Xie*, Hai-Ming Guo*, Catalytic asymmetric ring-opening of aminocyclopropanes with oxygen nucleophiles: access to chiral γ-amino acid derivatives, Org. Chem. Front., 2024, 11, 1817-1823.
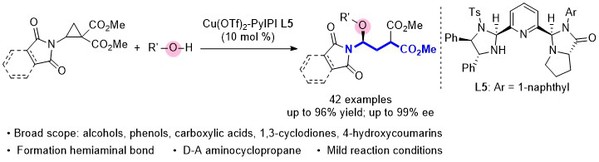
[12] Jun-Hao Zhang, Wei-Jing Yang, Ning Li, Yin Tian*, Ming-Sheng Xie*, Hai-Ming Guo*, Reversal of enantioselectivity in cobalt(II)-catalyzed asymmetric Michael–alkylation reactions: synthesis of spiro-cyclopropane-oxindoles, Org. Chem. Front., 2024,11, 4007-4013.
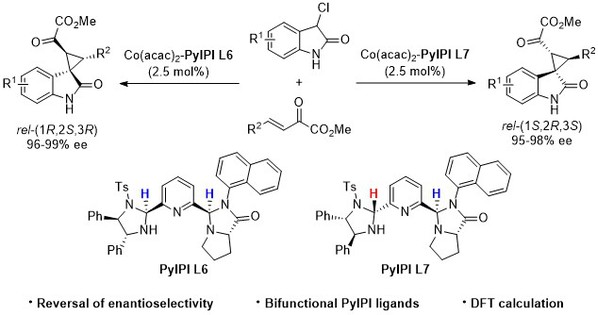
[13] Han-Le Wang, Tao Wei, Ru-Yun Bi, Ming-Sheng Xie*, Hai-Ming Guo*, Imidazolidine-Pyrroloimidazolone Pyridine-Ni(acac)2 Complex Catalyzed Asymmetric Darzens Reaction of Isatins with Phenacyl Chlorides: A Chiral Amplification Effect, Adv. Synth. Catal., 2024, 366, 14, 3188-3193.
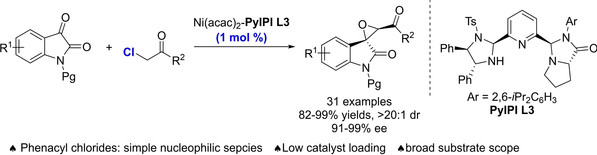
[14]Ya-Ping Wang, Xing-Ping Zhang, Ming-Sheng Xie*, Hai-Ming Guo*, Cobalt(II)-Catalyzed Enantioselective Propargyl Claisen Rearrangement: Access to Allenyl-Substituted Quaternary β-Ketoesters, Org. Lett., 2023, 25, 39, 7105-7109.
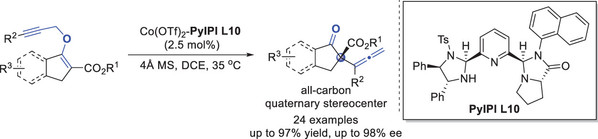
[15] Bai-Yu Xue, Chen-Ying Hou, Xiao-Bing Wang, Ming-Sheng Xie*, Hai-Ming Guo*, Asymmetric synthesis of 7-membered-ring-bridged 3,4-fused tricyclic indoles via Friedel–Crafts alkylation/annulation, Org. Chem. Front., 2023, 10, 1910-1914.
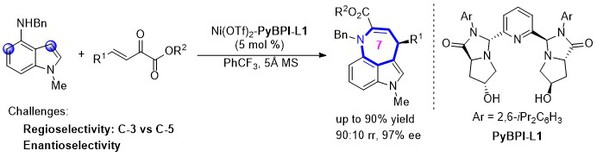
[16] Xiao-Bing Wang, Yin Tian, Li Zhou, Ming-Sheng Xie*, Gui-Rong Qu, and Hai-Ming Guo, Rational Design of Chiral Tridentate Ligands: Bifunctional Cobalt(II) Complex/Hydrogen Bond for Enantioselective Michael Reactions, Org. Lett. 2022, 24, 21, 3861–3866.
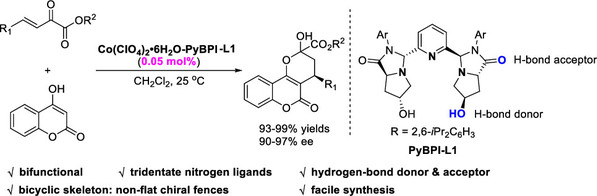
[17] Yang-Guang Chen, Heng-Bin Yu, Yin Tian*, Cheng Peng, Ming-Sheng Xie*, Hai-Ming Guo*, ArPNO-Catalyzed Acylative Dynamic Kinetic Resolution of 3-Hydroxyphthalides: Access to Enantioenriched Phthalidyl Esters, Org. Lett. 2023, 25, 5585-5590. 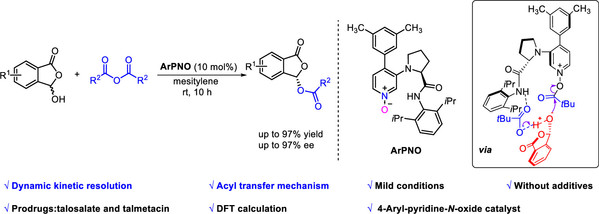
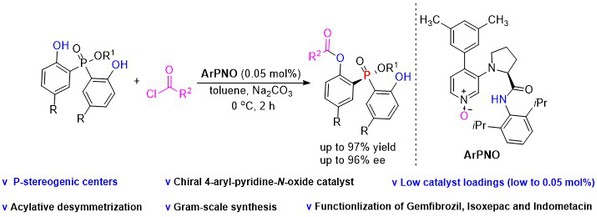
[18] Yang-Guang Chen, Ying Hu, Jia-Yi Liu, Ming-Sheng Xie*, Hai-Ming Guo*, Pyridine-N-oxide catalyzed acylative desymmetrization of bisphenols: access to P-stereogenic phosphinates with low catalyst loadings, Org. Chem. Front., 2023, 10, 6140-6145.
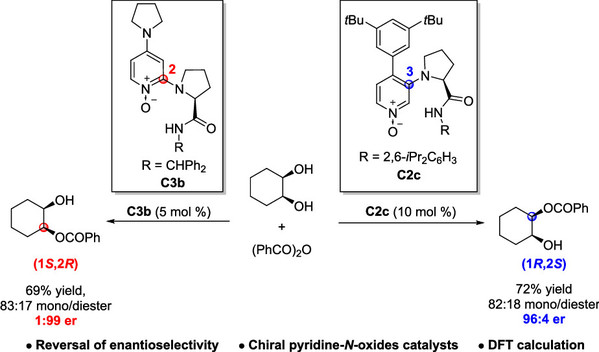
[19] Sai-Ya Lian, Ning Li, Yin Tian*, Cheng Peng, Ming-Sheng Xie*, Hai-Ming Guo, Reversal of Enantioselectivity for the Desymmetrization of meso-1,2-Diols Catalyzed by Pyridine-N-oxides, J. Org. Chem. 2023, 88, 13771-13781.
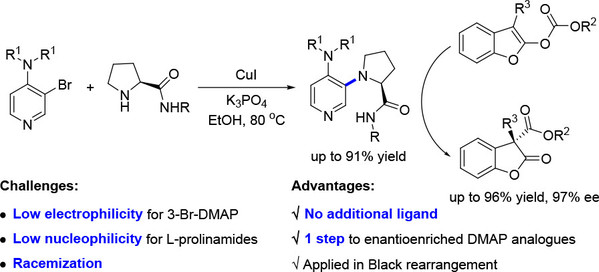
[20] Lei Cao, Xing-Ping Zhang, Ming-Sheng Xie,* and Hai-Ming Guo Cu-Catalyzed N-Arylation of Prolinamides: Access to Enantioenriched DMAP Analogues and Its Application in Black Rearrangement, J. Org. Chem.2023, 88(1), 341-346.
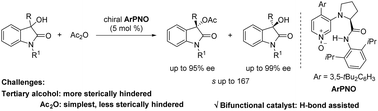
[21] Min Yang, Yu-Lin Gao, Ming-Sheng Xie,* and Hai-Ming Guo* ArPNO-catalyzed acylative kinetic resolution of tertiary alcohols: access to 3-hydroxy-3-substituted oxindoles, Org. Biomol. Chem., 2022, 20(32), 6351-6355.
[22] Meng Shan, Tao Liang, Ye-Fei Zhang, Ming-Sheng Xie,* Gui-Rong Qu, and Hai-Ming Guo* Enantioselective rearrangement of indolyl carbonates catalyzed by chiral DMAP-N-oxides, Org. Chem. Front. 2019, 6(23), 3874-3878.
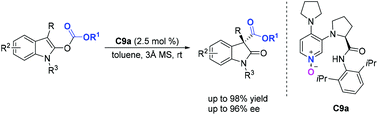
[23]Huifang Zhang, Mingsheng Xie,* Guirong Qu, and Junbiao Chang* Dynamic Kinetic Resolution of α-Purine Substituted Alkanoic Acids: Access to Chiral Acyclic Purine Nucleosides, Org. Lett. 2019, 21(1), 120-123. 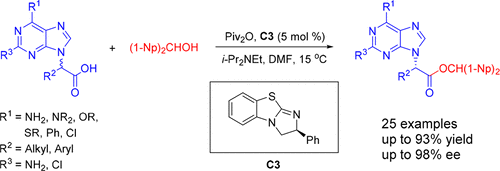
[24] Ming-Sheng Xie,* Yang-Guang Chen, Xiao-Xia Wu, Gui-Rong Qu, and Hai-Ming Guo* Asymmetric Synthesis of Chiral Acyclic Purine Nucleosides Containing a Hemiaminal Ester Moiety via Three-Component Dynamic Kinetic Resolution, Org. Lett. 2018, 20(4), 1212-1215. 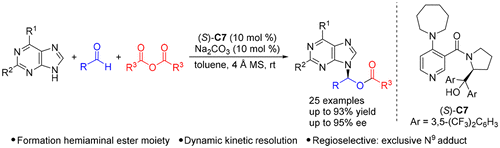
[25] Ming-Sheng Xie, Guo-Feng Zhao, Tao Qin, Yong-Bo Suo, Gui-Rong Qu, and Hai-Ming Guo*, Thiourea participation in [3+2] cycloaddition with donor–acceptor cyclopropanes: a domino process to 2-amino-dihydrothiophenes, Chem. Commun. 2019, 55(11), 1580-1583.
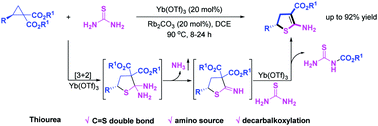
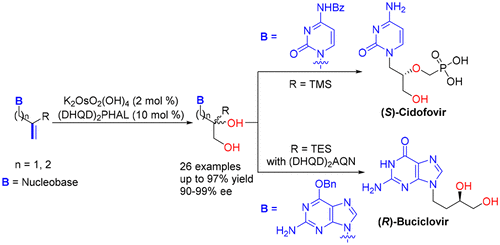
[26] Tao Qin, Jian-Ping Li, Ming-Sheng Xie,* Gui-Rong Qu, and Hai-Ming Guo*, Synthesis of Chiral Acyclic Nucleosides by Sharpless Asymmetric Dihydroxylation: Access to Cidofovir and Buciclovir, J. Org. Chem.2018, 83(24), 15512-15523.
[27] Ming-Sheng Xie,* Xuan Cheng (成轩,本科生), Yang-Guang Chen, Xiao-Xia Wu, Gui-Rong Qu, and Hai-Ming Guo*, Efficient synthesis of tetrazole hemiaminal silyl ethers via three-component hemiaminal silylation, Org. Biomol. Chem. 2018, 16(38), 6890-6894. 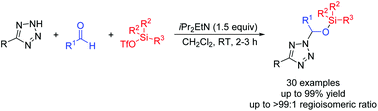
[28] Mingsheng Xie, Xiaohua Liu, Xiaoxia Wu, Yunfei Cai, Lili Lin and Xiaoming Feng*, Catalytic Asymmetric [8+2] Cycloaddition: Synthesis of Cycloheptatriene-Fused Pyrrole Derivatives,Angew. Chem. Int. Ed. (2013), 52, 5604-5607. [29] Mingsheng Xie, Xiaohong Chen, Yin Zhu, Bo Gao, Lili Lin, Xiaohua Liu and Xiaoming Feng*,Asymmetric Three-Component Inverse Electron-Demand Aza-Diels–Alder Reaction: Efficient Synthesis of Ring-Fused Tetrahydroquinolines,Angew. Chem. Int. Ed. (2010), 49, 3799-3801. [30] 谢明胜, 武晓霞, 王刚, 林丽丽, 冯小明*, 催化不对称[8+2]环加成反应构建环庚三烯并吡咯烷-3,3’-吲哚酮,化学学报,2014,72, 856-861. [31] Mingsheng Xie, Xiaohua Liu, Yin Zhu, Xiaohu Zhao, Yong Xia, Lili Lin and Xiaoming Feng*, Asymmetric Synthesis of Tetrahydroquinolines with Quaternary Stereocenters via Povarov Reaction, Chemistry–A European Journal (2011), 17, 13800-13805.
|